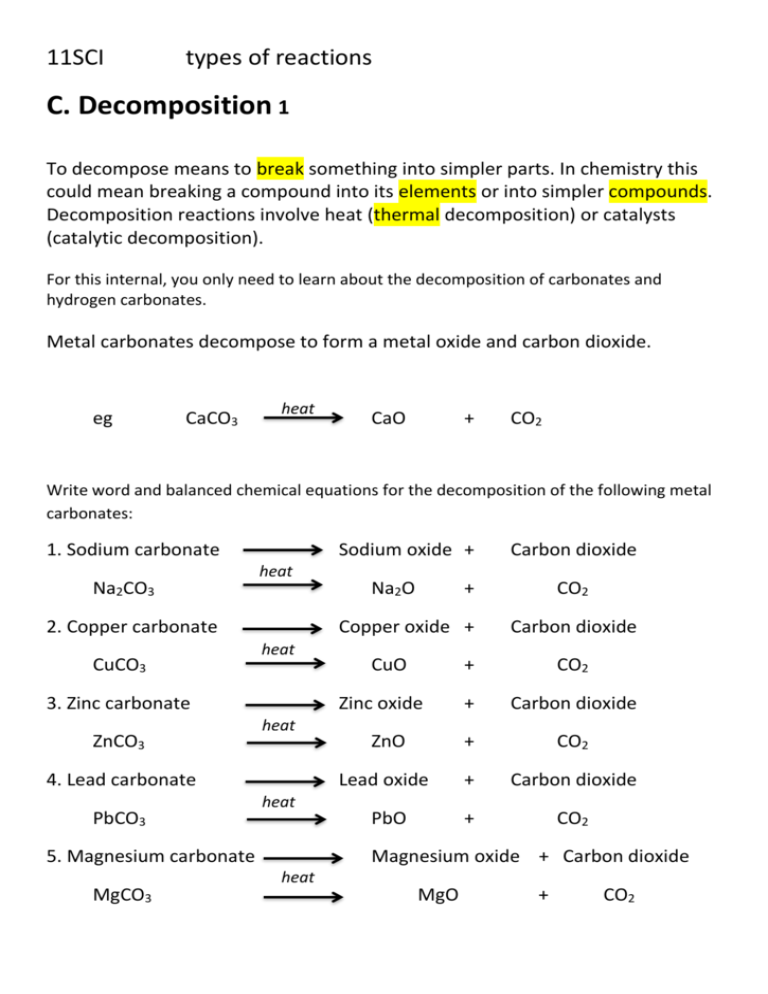
Write balanced chemical equation for the reactions taking place when (i) Zinc carbonate is calcinated. (ii) Zinc sulphide is roasted or heated in air. (iii) Zinc oxide is reduced to zinc.

Question Video: Identifying the Correct Chemical Equation for the Decomposition of Zinc Carbonate | Nagwa

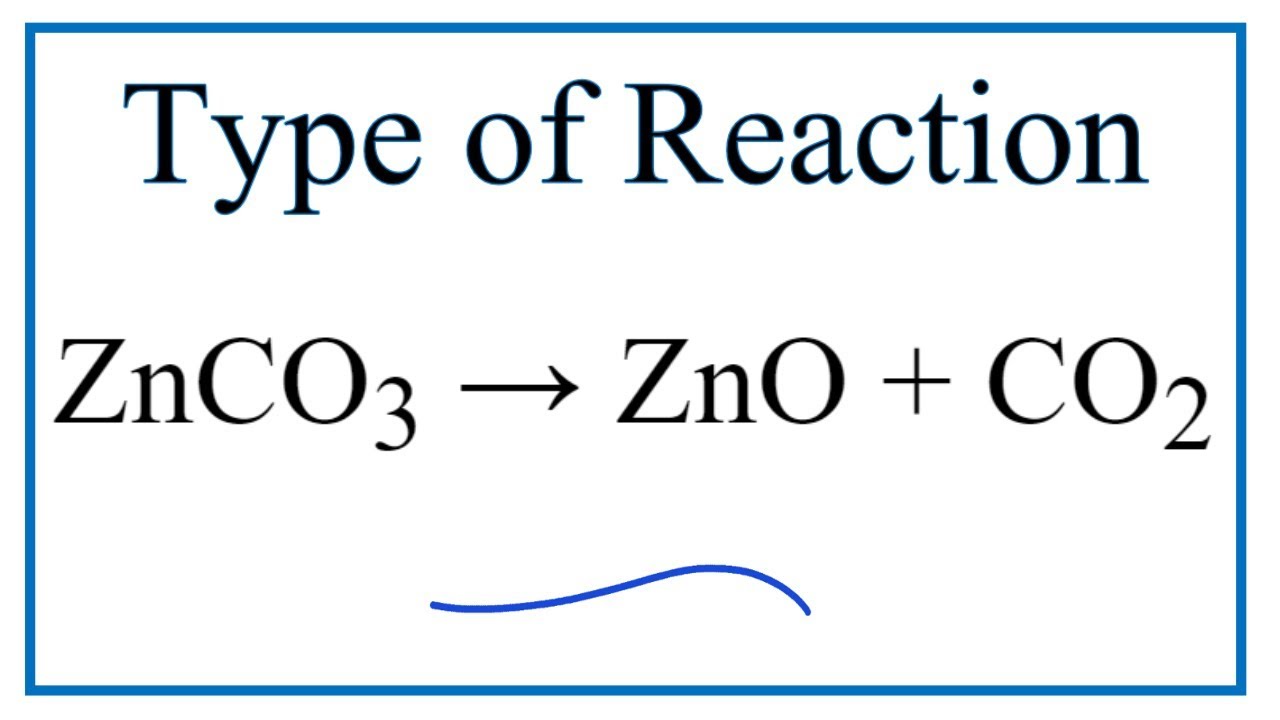
Write the balanced chemical equations for the following and identify the type of reaction in each - YouTube

Zinc oxide reacts whith carbon, on heating, to from zinc metal and carbon monoxide. Write a balanced - YouTube

ZnCO3=ZnO+CO2 balance the chemical equation. znco3=zno+co2 zinc carbonate is gives us zinc oxide - YouTube

Zinc carbonate(s) → Zinc oxide (s) + Carbon dioxide (g) bromide(s) write the balance equation - Brainly.in

Write the balanced chemical equation for the following and identify the type of reaction in each case : Zinc carbonate (s) to Zinc oxide (s) + Carbon dioxide (g)

Write chemical reaction for each- (1) Zinc carbonate is calcinated. (2) cinnabar is heated in the air. - Brainly.in
Calculate the volume of carbondioxide formed at STP and the weight or zinc oxide formed in grams , when 2 . 32 g of zinc carbonate decomposes with no further loss in weight ?

Basic zinc carbonate as a precursor in the solvothermal synthesis of nano-zinc oxide - ScienceDirect
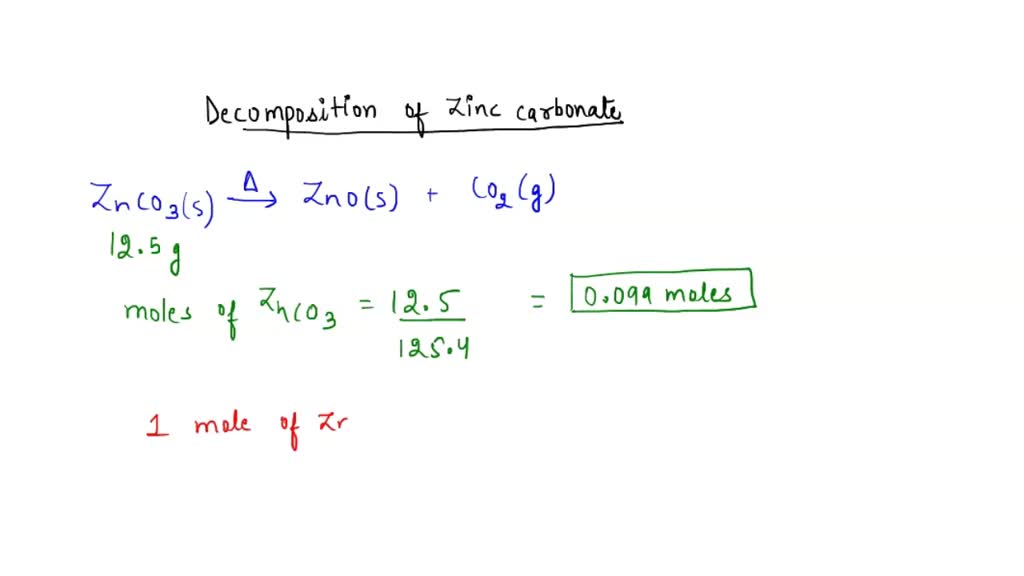
SOLVED: 12.5g of zinc carbonate is heated, it decomposes to make 8.1g of zinc carbonate. Calculate the mass of carbon dioxide made.