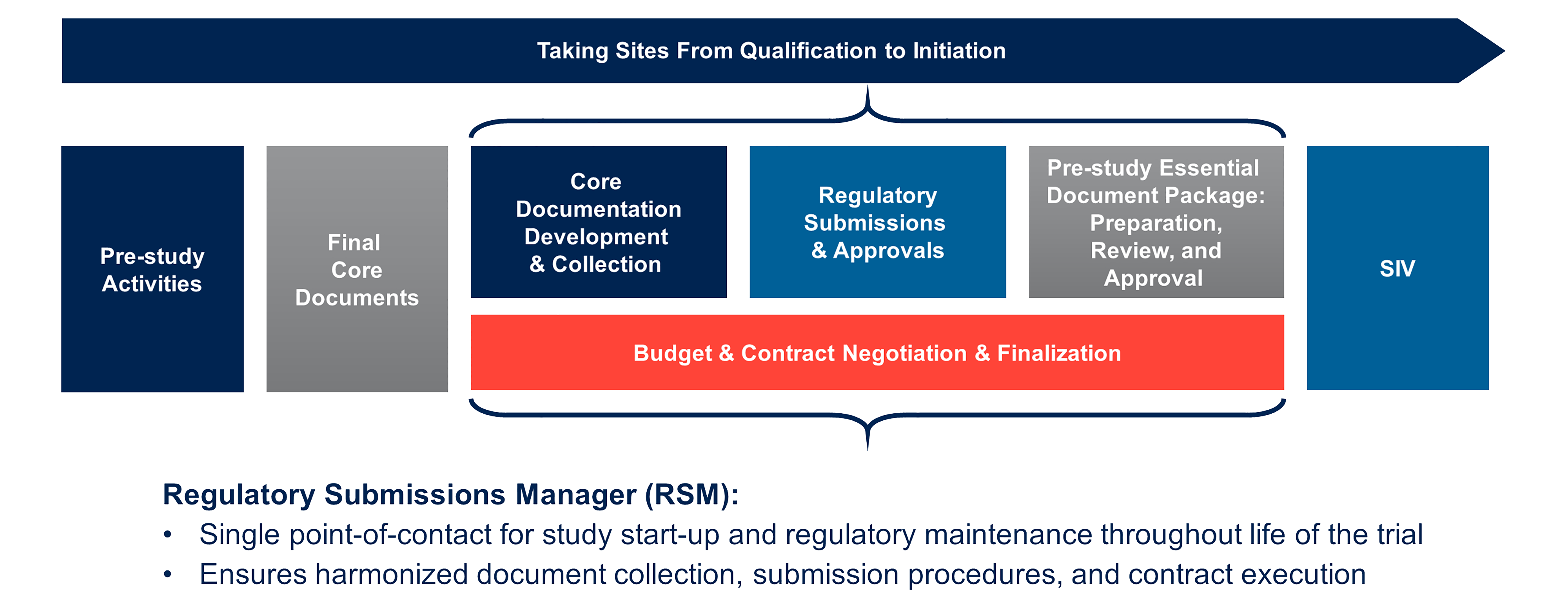
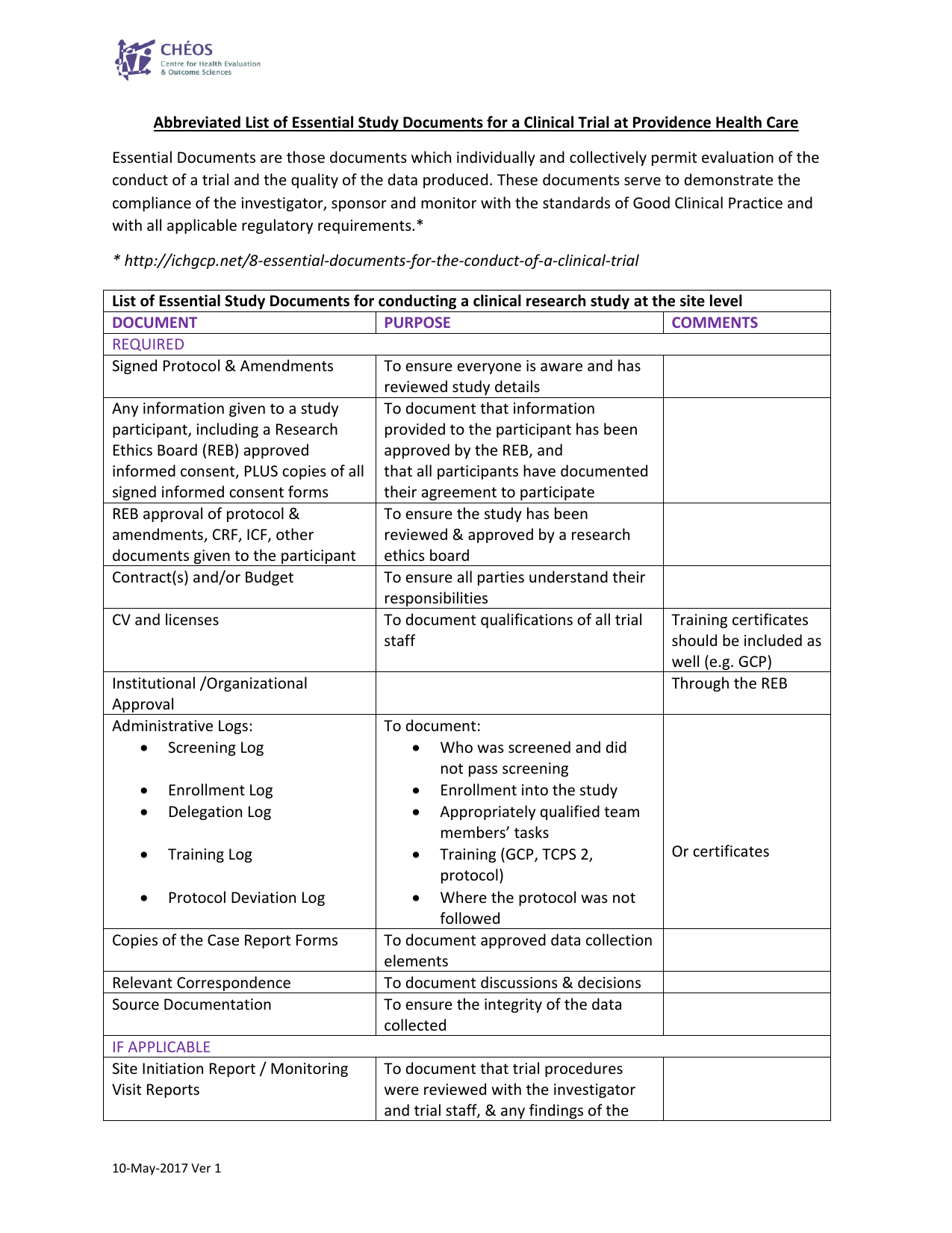
Nova Southeastern University Standard Operating Procedure for GCP Title: Essential Documents for a Clinical Trial Version # 1 S

PPT - Nilvad GCP Overview Susan Lennon Clinical Research Manager ICRIN/MMI PowerPoint Presentation - ID:2751242

Research2note on Twitter: "The what, when and why of research essential documents @NHSRDForum @dollyblue3 @DigitalCRN @DarkNatter @Research_Innov @WeNurses https://t.co/yPlmOQEHc6" / Twitter

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health