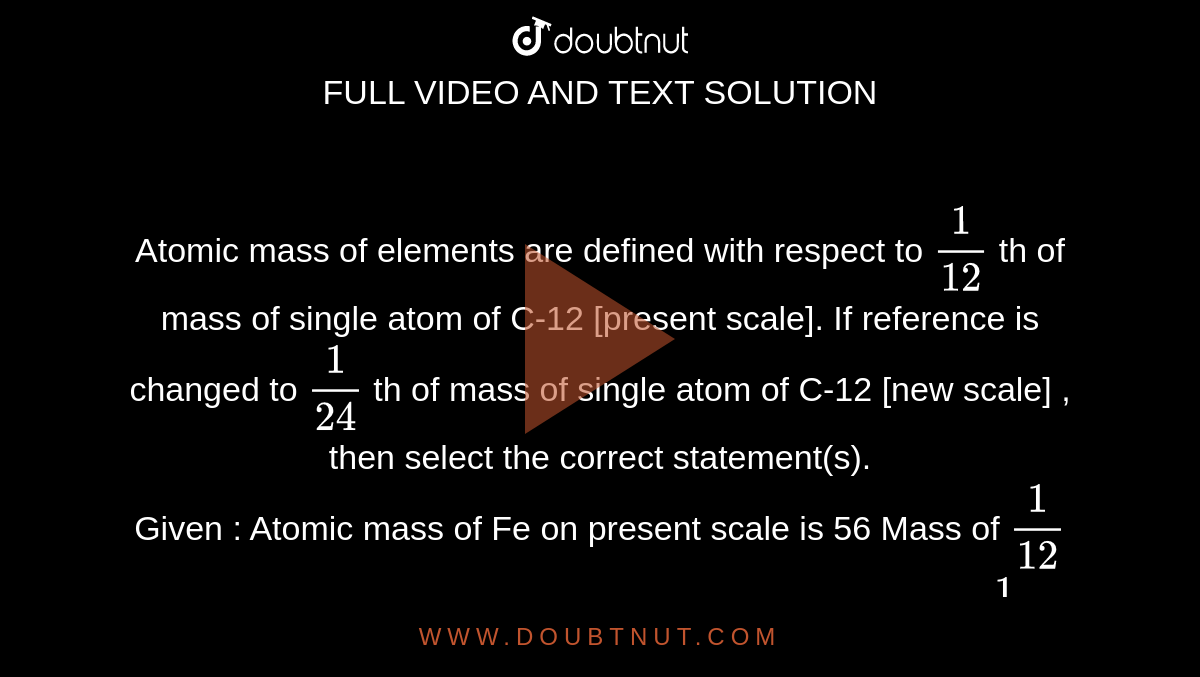
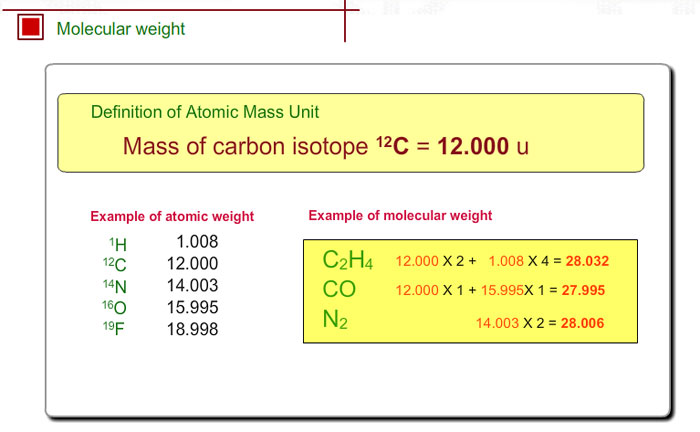
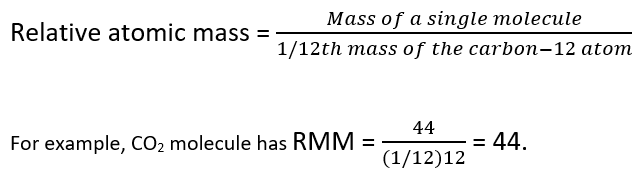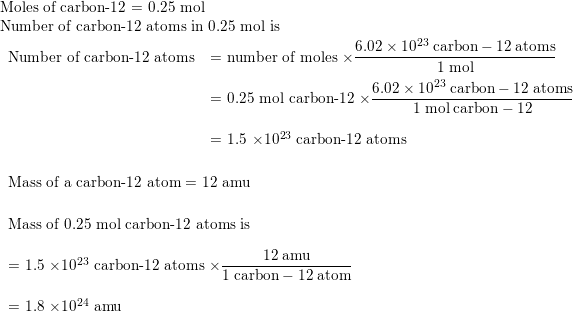What is 1/12 of the mass of a carbon 12 atom? And why do we compare atomic masses of other elements with respect to it? - Quora

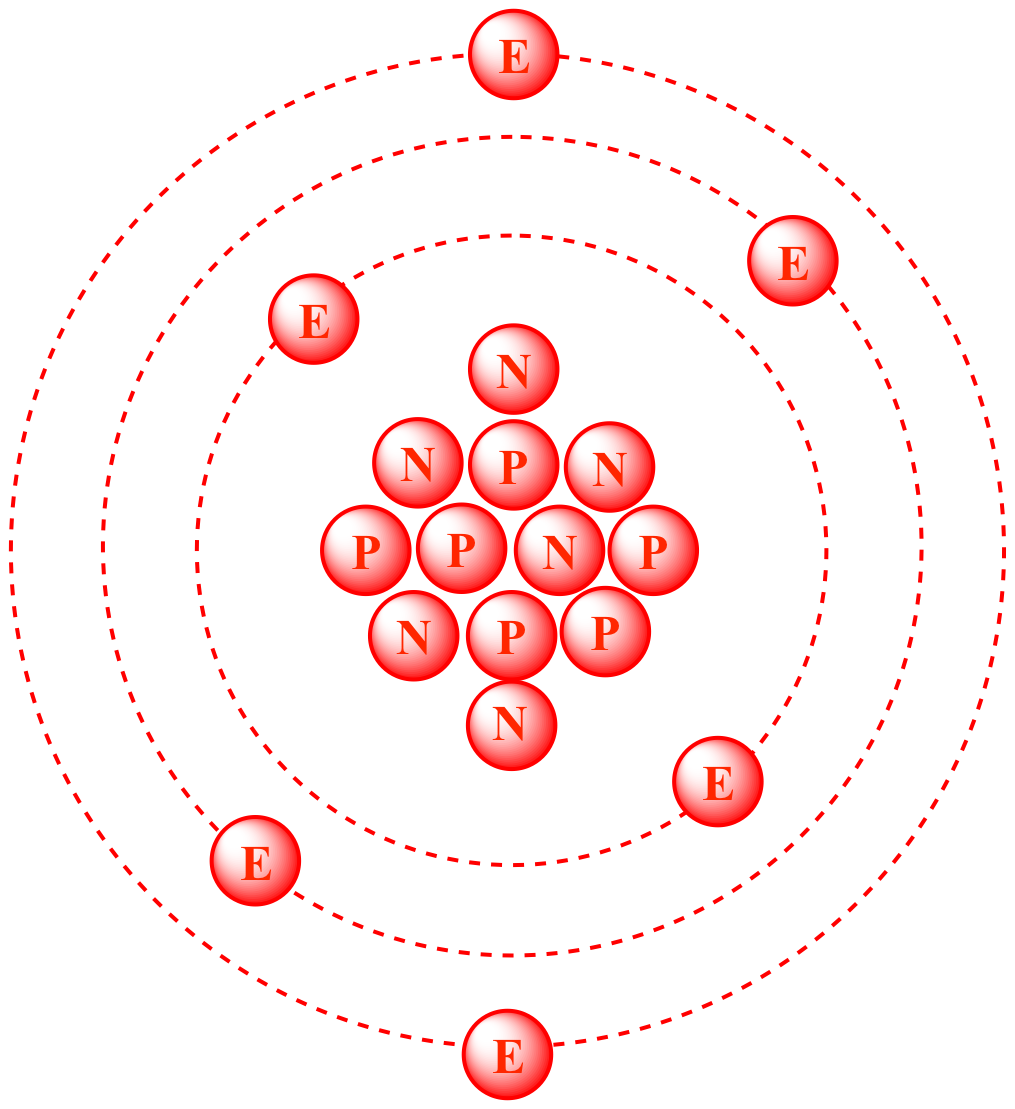
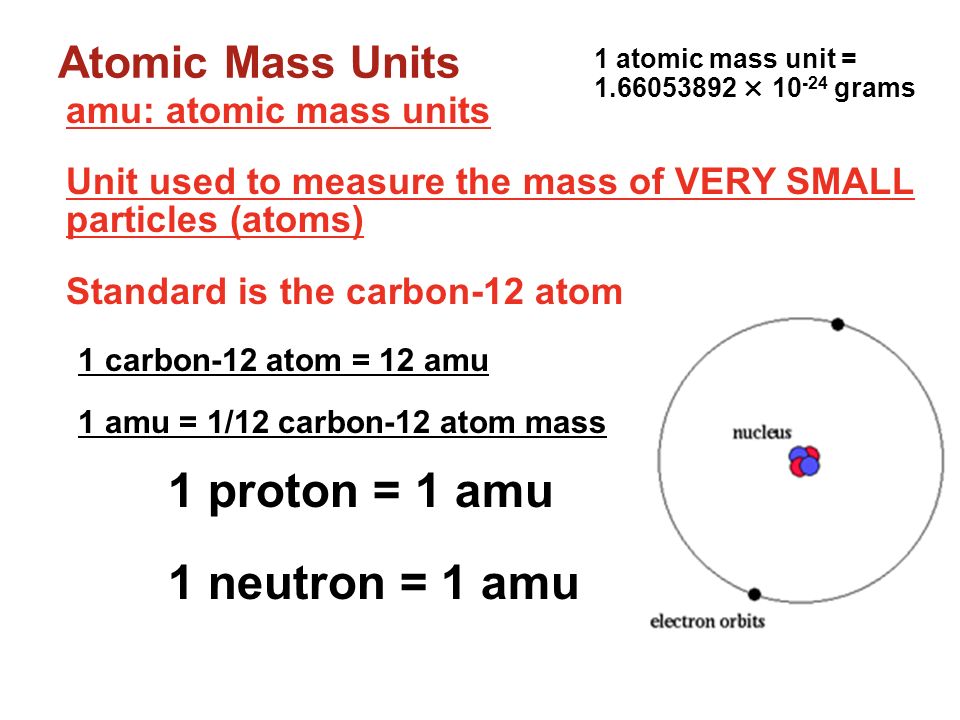
Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download

If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of 1 atom of carbon?... - YouTube

if we consider that 1/6, in place of 1/12, mass of carbon atom is taken to be relative atomic mass - Chemistry - Some Basic Concepts of Chemistry - 13772503 | Meritnation.com

Atomic Mass Unit: amu (atomic mass unit) amu is defined as a mass exactly equal to on-twelfth the mass of Carbon-12 atom amu = 1/12 of carbon-12 Hydrogen. - ppt download

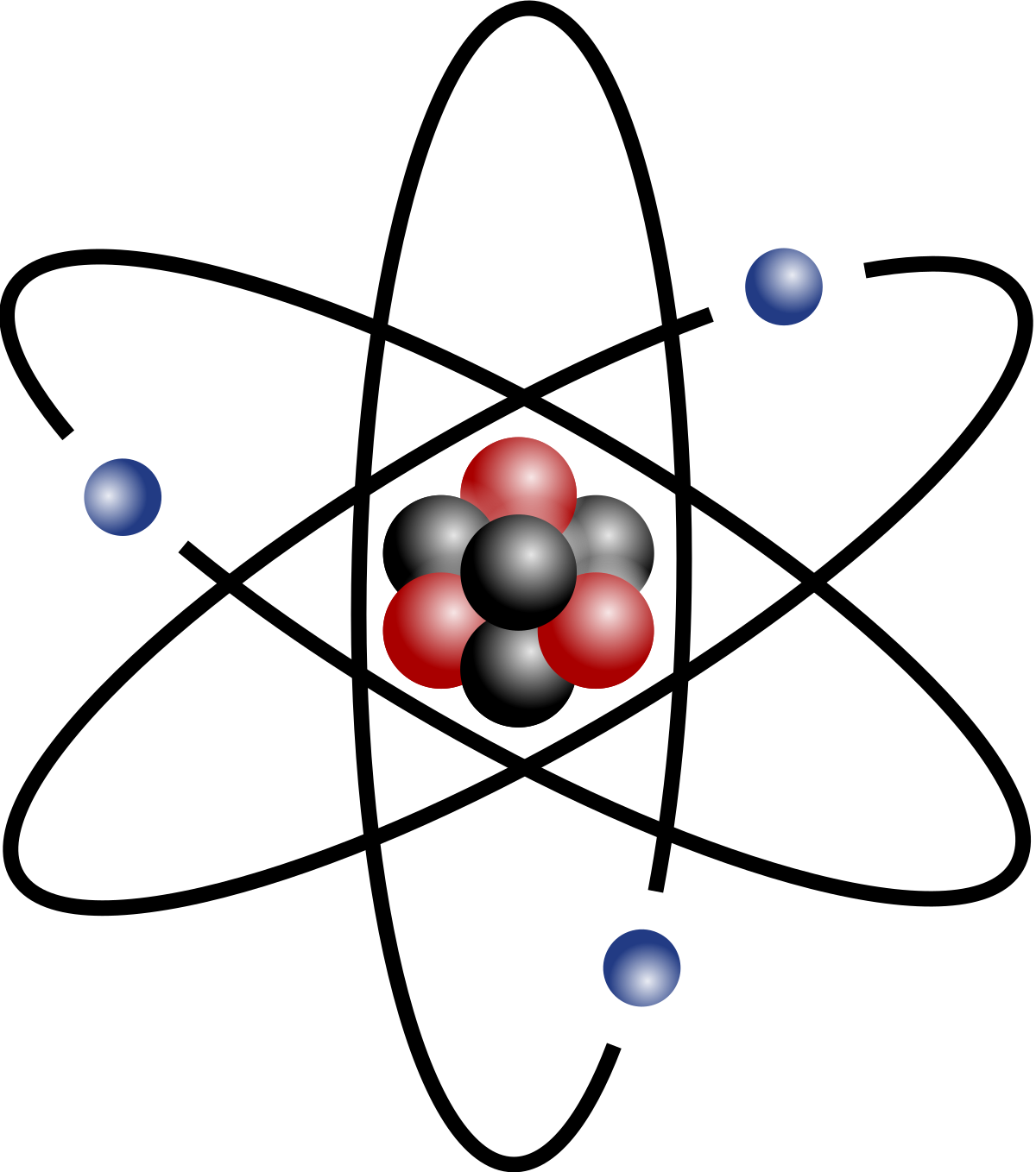
SOLVED: The atomic mass unit (amu) is defined as 1/12 the mass of a carbon- 12 atom. Therefore, a carbon atom with 6 protons and 6 neutrons has a mass of exactly 12